Carbapenemase Challenges and Solutions in Europe
Watch Now
Hear from leading experts on recent challenges and solutions for carbapenemase-producing Enterobacteriaceae (CPE) in France and Italy. Scroll down to listen to brief interviews with:
- Thierry Naas – French National Reference Center for Antibiotic Resistance
- Gian Maria Rossolini – Careggi University Hospital and Claudio Macchi – Don Gnocchi Foundation
Clinical and Economic Benefits of Implementing a Molecular Testing Algorithm for TB
One of the most feared consequences of a delayed tuberculosis (TB) diagnosis is nosocomial transmission of TB. The CDC’s guidelines to help limit the spread of this infection by keeping a patient isolated until highly infectious strains of TB can be excluded are resource intensive. For this reason, time to diagnosis carries significant weight.
A recent study analyzed the feasibility, safety, and clinical impact of utilizing molecular testing strategies to guide discontinuation of respiratory isolation among hospitalized patients undergoing evaluation for active TB. The researchers used Cepheid’s GeneXpert MTB/RIF* test, which provides results in less than 2 hours.
They concluded that using molecular testing was associated with a significant reduction in time to isolation discontinuation and hospital discharge, as well as cost savings, compared to a conventional, microscopy-based testing strategy.
Together, these measures of impact place rapid molecular testing for TB among a select group of interventions that have been shown to advance the “quadruple aim”: improved population health, a better patient experience, a better clinician experience, and lower costs."
Epidemiologist, John Hopkins Bloomberg School of Public Health
Chaisson LH, et al. Association of Rapid Molecular Testing With Duration of Respiratory Isolation for Patients With Possible Tuberculosis in a US Hospital. JAMA Intern Med. 2018 Aug 27; doi:10.1001/jamainternmed.2018.3638. [Epub ahead of print].
*For in vitro Diagnostic Use
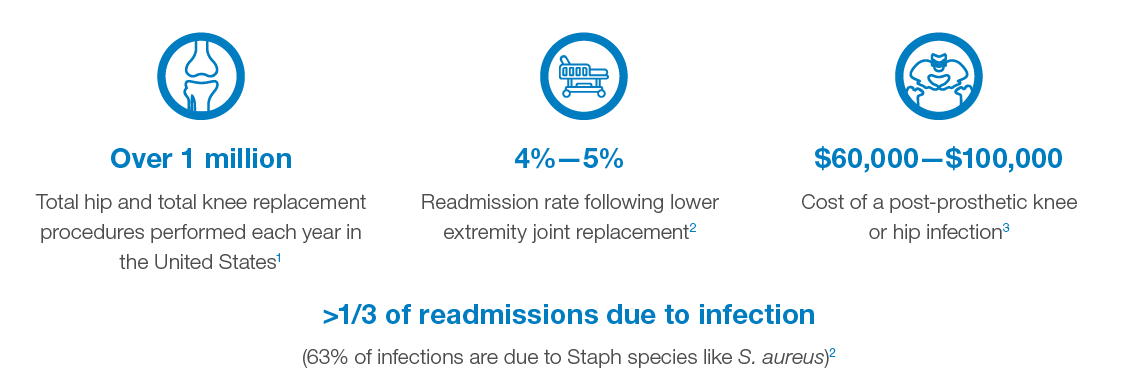
Impact of MRSA/S. aureus Colonization on Surgical Site Infections
Reduce Joint Replacement Surgical Site Infections (SSIs) with Molecular Testing
Colonized patients are up to 9 times more likely to develop an SSI,1 and more than 8 out of 10 cases of S. aureus bacteremia are believed to be caused by a patient’s own flora.2,3 Knowing if a patient is colonized with MRSA or S. aureus can enable targeted infection control practices prior to surgery. However, traditional culture techniques may miss MRSA colonization in up to a third of the cases.4,5
CLICK HERE to download the SSI infographic.
For In Vitro Diagnostic Use.
References:
1. Kluytmans J, et al. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-20
2. von Eiff C, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11-6.
3. Critchley IA. Eradication of MRSA nasal colonization as a strategy for infection prevention. Drug Discov Today Ther Strateg. 2006;3:189-95.
4. Wisniewski, TR. Comparison of Bio-Rad MRSA Select Agar with BBL ChromAgar for MRSA Nares Swab Surveillance Cultures in VISN 12. ASM2008;C-130.
5. Nahimana I, et al. Evaluation of three chromogenic media (MRSA-ID, MRSA-Select and CHROMagar MRSA) and ORSAB for surveillance cultures of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2006; 12:1168-74.
One Mother’s Perspective – Real-Life Impact of Antimicrobial Resistance
Watch Now
Everly Macario has her doctorate in public health from Harvard University, but was largely unfamiliar with the threat of antimicrobial resistance until it impacted her family in a most personal, painful way. Everly’s story underscores the imperative need for rapid diagnostics in our healthcare system in order to prevent unnecessary antibiotic treatment and the dire repercussions it has on both an individual and global scale.
Presurgical Molecular Testing for Staphyoccocal Nasal Colonization
A Targeted Approach to Reducing Surgical Site Infections
|
Routine molecular testing of the nares for the presence of Staphylococcus aureus followed by targeted decolonization of patients carrying either S. aureus (SA) or Methicillin-resistant S. aureus (MRSA) prior to surgery is an effective strategy for preventing surgical site infections (SSIs).1,2,3 It is consistent with both infection prevention and antimicrobial stewardship efforts. This article provides an overview of the value of presurgical molecular testing and targeted decolonization at reducing post–joint replacement SSIs. CLICK HERE to download the white paper. |
References:
1. Bode LG, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9-17.
2. Jain R, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419-30.
3. van Rijen MML, et al. Reduced Costs for Staphylococcus aureus Carriers Treated Prophylactically with Mupirocin and Chlorhexidine in Cardiothoracic and Orthopaedic Surgery. PLoS One. 2012;7:e43065.
Active Surveillance Testing with Targeted MRSA Decolonization
A Cost-Effective Way for Infection Preventionists to Stem Outbreaks and Practice Good Antimicrobial Stewardship
|
There are two main approaches that hospitals and other healthcare organizations are taking to prevent MRSA infections, particularly among their most vulnerable patient populations. These populations include patients admitted to intensive care units (ICUs), those undergoing surgery, and those transferred from another healthcare facility. The approaches are:
Which method is more effective at preventing MRSA outbreaks in a healthcare facility, and at what cost? CLICK HERE to download the white paper. |
In order to reduce additional selection pressure in [hospital-acquired] pathogens it seems to make sense to restrict the valuable agent CHG to those indications with a clear patient benefit and to eliminate it from applications without any benefit or with a doubtful benefit."
References:
Kampf G. Acquired resistance to chlorhexidine—is it time to establish an ‘antiseptic stewardship’ initiative? Journal of Hospital Infection. 2016; 1-15.
Preventing "Nightmare Bacteria" from Becoming Nightmare Outbreaks
|
In early 2015, carbapenem-resistant Enterobacteriaceae (CRE) became a household term when a high-profile outbreak — including two deaths — at a California hospital were linked to contaminated medical scopes.1 But these multidrug-resistant pathogens have been a growing problem for at least a decade. CRE infections increased five-fold from 2008 to 2012, reaching 48 states by 2012.2 In 2012, the U.S. Centers for Disease Control and Prevention (CDC) reported that approximately 4% of acute care and 18% of long-term acute care hospitals in the U.S. reported at least one patient with a CRE infection during the first half of that year.3 Now, in 2017, an Infection Control and Hospital Epidemiology study of 16 healthcare facilities in the Washington, D.C. area reported a surprisingly high 5.2% prevalence rate.4 That’s only one metropolitan area of the U.S., but it’s certainly not unique. CLICK HERE to download the white paper. |
1. Terhune C. Superbug linked to 2 deaths at UCLA hospital; 179 potentially exposed. Los Angeles Times. 2015 Feb 18. Accessed Oct 2017. http://www.latimes.com/business/la-fi-hospital-infections-20150218-story.html
2. Thaden JT, et al. Rising rates of carbapenem-resistant Enterobacteriaceae in community hospitals: a mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the Southeastern United States. Infect Control Hosp Epidemiol. 2014 Aug;35(8):978-83.
3. CDC. CDC: Action needed now to halt spread of deadly bacteria. Press release. 2013 Mar 5. Accessed Oct 2017. https://www.cdc.gov/media/releases/2013/p0305_deadly_bacteria.html
4. Reuben J, et al; HARP Study Team. Healthcare antibiotic resistance prevalence - DC (HARP-DC): a regional prevalence assessment of carbapenem-resistant Enterobacteriaceae (CRE) in healthcare facilities in Washington, District of Columbia. Infect Control Hosp Epidemiol. 2017 Aug;38(8):921-29.
Routine Molecular Surveillance Testing for CRE in a Low-Prevalence Setting
A study evaluating patients at high risk of being carriers of carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CRE) demonstrated that routine surveillance testing may effectively guide infection control programs to limit the spread of CRE, even in low-prevalence areas.
While early detection of patients colonized with carbapenemase-producing CRE is crucial for infection control, there are no clear recommendations on when to use molecular methods for active surveillance in low-prevalence settings. This study demonstrated that a rapid molecular test is well adapted for routine surveillance testing of high-risk patients even in low-prevalence regions: It found Cepheid’s Xpert® Carba-R test to be 100% sensitive and 99.13% specific for the detection of carbapenemase-producing CRE from rectal swab samples, compared to chromogenic culture after enrichment.
The study was conducted at a hospital in France where carbapenemase-producing CRE prevalence is <1%. The “high risk patients” included those repatriated from countries known for high CRE prevalence or contact patients of a known carbapenemase-producing CRE carrier. The study allowed rapid cohorting of carriers, which is crucial (i) to prevent outbreaks and (ii) to reduce the social impact and the cost of infection control measures empirically implemented for high-risk patients who end up being negative for colonization with carbapenemase-producing CRE.
The report of the findings was published in International Journal of Antimicrobial Agents.
This study demonstrated that Xpert® Carba-R is well adapted for rapid [surveillance testing] of high-risk patients even in low prevalence regions.”
Hoyos-Mallecot et al. Int J Antimicrob Agents 49 (6), 774-777. 2017 Apr 12.
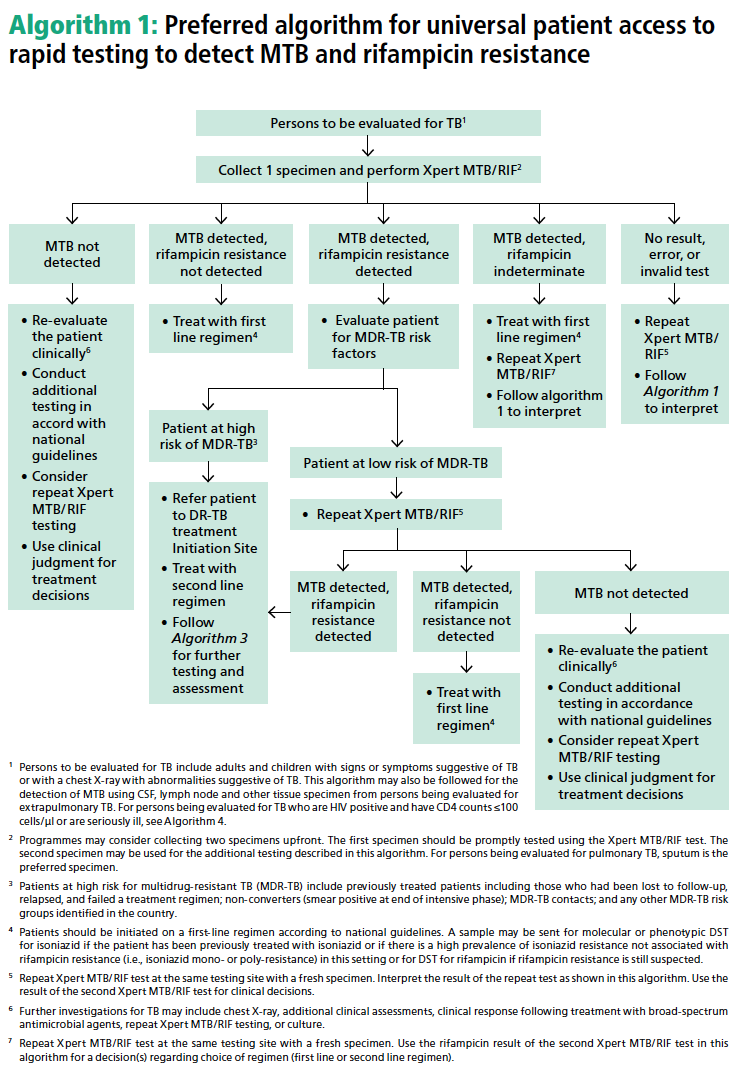
Global Laboratory Initiative Outlines Protocol for Finding Early Active Cases of TB
Laboratories play a crucial role in identifying early active cases of Tuberculosis (TB), which is a key to eliminating the disease worldwide. In order to meet WHO’s goals for prevention of TB transmission and improvement of patient care, rapid testing of TB suspects with sensitive molecular diagnostic tests is recommended by the Global Laboratory Initiative (GLI) and experts from the European Tuberculosis Laboratory Initiative (WHO European Region). Both global expert groups recommend the use of Cepheid’s Xpert MTB/RIF as a frontline test, rather than smear microscopy or line probe assays in conjunction with culture-based methods for drug susceptibility testing.1,2
In its publication, GLI outlined a preferred algorithm for promoting universal patient access to rapid testing for TB and rifampicin resistance.1 Read more about the GLI model by clicking here.
1. Global Laboratory Initiative. GLI model TB diagnostic algorithms. Accessed Mar 2017. http://www.stoptb.org/wg/gli/assets/documents/GLI_algorithms.pdf
2. European Tuberculosis Laboratory Initiative. Algorithm for laboratory diagnosis and treatment-monitoring of pulmonary tuberculosis and drug-resistant tuberculosis using state-of-the-art rapid molecular diagnostic technologies. Accessed Mar 2017. http://www.euro.who.int/__data/assets/pdf_file/0006/333960/ELI-Algorithm.pdf
Complex transmission pathways of VRE necessitate active screening
A 7-year study of the routes of nosocomial vancomycin-resistant Enterococcus (VRE) transmission in a single institute in Cambridge, United Kingdom, provides new information with important implications for hospital infection control. The study identified multiple routes of VRE introduction into the hospital followed by a complex network of organism transmission thereafter.
These findings reinforce the importance of using active screening to detect VRE carriers in conjunction with infection control measures to prevent further transmission. Active screening as part of a broader infection prevention program can reduce the rates of VRE colonization and infection, which can lead to lower rates of infection such as seen in countries that implement this strategy, such as Finland and the Netherlands.
This study used patient admission and ward movement data along with advanced whole genome sequencing methods to track introduction and transmission of multiple VRE strains. More than 50% of isolates found in the study were highly related to at least one other isolate, highlighting the complexity of VRE transmission within the hospital. Given the data, the investigators concluded that targeted infection control interventions triggered only by outbreak investigations, without active screening, would only be partially effective in reducing VRE rates.
Active screening reduces the rates of VRE infection, and countries that implement this strategy (eg, Finland and the Netherlands) have considerably lower rates of VRE infection than the United Kingdom.”
VRE is a leading cause of healthcare-associated infections. These infections, compared with those caused by vancomycin-susceptible enterococci, have higher mortality rates and healthcare costs. Because it can often persist in hospital settings, vancomycin resistant strains of Enterococcus faecium is a particular challenge to eradicate. This challenge is compounded by a lack of active screening.
The report of their findings was published in Clinical Infectious Diseases.
KE Raven et al. Clinical Infectious Diseases 2017
Benefits of testing for S. aureus before surgery highlighted in consensus paper
The benefits of using rapid molecular diagnostic technology for pre-operative screening of MRSA/S. aureus carriers are highlighted by an international group of infection control experts in a recent consensus paper.
Surgical site infections (SSIs) are among the most common healthcare-associated infections (HAIs), and result in significant morbidity and mortality. Nasal colonization with S. aureus is the most important independent risk factor for the development of an SSI in clean surgery, as the rate is 2 to 9 times higher in carriers than in non-carriers.
Following the 1st European S. aureus & Surgical Site Infection round table, 10 European and American experts reviewed the evidence on the value of screening for nasal carriage of S. aureus and subsequent decolonization of positive patients pre-operatively. Their findings were published as a consensus paper in the Journal of Hospital Infection.
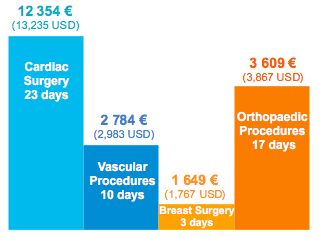
SSI Additional Length of Stay and Cost
There is a clear correlation between empirical/universal use of mupirocin and antiseptic and the emergence of resistance to these agents amongst MSSA, MRSA and S. epidermidis, increasing from 60 to 95%.
An efficient solution for patients undergoing clean surgery is the utilization of rapid molecular diagnostic technology for the pre-operative identification of carriers of MSSA and MRSA, followed by mupirocin and chlorhexidine decolonization.
This treatment strategy is associated with reduced SSI rates and cost savings:
- Reduced the rate of S. aureus infection by 60%
- Decreased the mortality rate by 57%
- Reduced hospital LOS by 2 days
- Reduced cost of care by 2 841 € (3,044 USD) per cardiothoracic patient and 955 € (1,023 USD) per orthopedic patient
Screening and selective decolonization of patients positive for S. aureus have the benefits of preventing SSIs, helping to contain costs, monitoring changes in circulating isolates of MSSA and MRSA, and minimizing the emergence of resistance.”
Reference:
H. Humphreys et al. Journal of Hospital Infection 2016
Carbapenemases: The UK Experience
Watch Now
Hear from leading UK experts on recent challenges and solutions with carbapenemase-producing Enterobacteriaceae (CPE) in northwest England.
The UK has been leading the way with innovative approaches to address the carbapenemase threat and limit the spread of these dangerous organisms. Scroll down to listen to short interviews with key experts in the field:
- Dr. Tim Neal – Infection Control Doctor – Liverpool Clinical Laboratories
- Dr. Andrew Dodgson – Microbiology and Infection Control Lead – Central Manchester Foundation Trust
- Terry Whalley – Director of Strategy & Sustainability, Wirral University Teaching Hospital NHS Foundation Trust
Effectiveness of MRSA Surveillance Highlighted in Update to Landmark Study
The American Journal of Infection Control published a study out of the Veterans Health Administration (VA Health System) — the largest integrated health care system in the US — highlighting its continued success reducing MRSA rates system-wide using a program centered around on-demand PCR surveillance testing.1 Over an 8-year period, the initiative is credited with reducing MRSA healthcare-associated infection (HAI) rates by:
- 87% in ICUs
- 80% in non-ICU settings
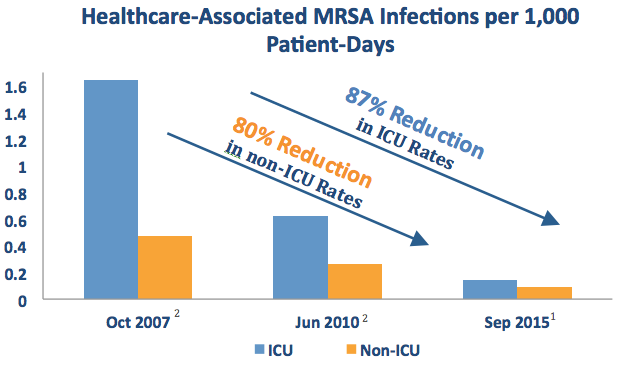
As noted in a separate publication in 2016,3 the VA also deemed this initiative to be cost effective.
The authors of the most recent article credit active surveillance testing with playing a critical role in their long-term success reducing MRSA rates:
We speculate that active surveillance was the primary driver of the downward trends seen in the VA because MRSA HAI rates had not changed before October 2007 when the Initiative was fully implemented, even though formal recommendations for hand hygiene and device-related infection control bundles had been in place for several years.”
Click here to read the full open source article.
References:
1. Evans ME et al. Am J Infect Control. 2017;45:13-6.
2. Jain R et al. N Engl J Med. 2011;364:1419-30.
3. Nelson RE et al. Am J Prev Med. 2016;50(5S1):S58-S65.
The Role of Molecular Diagnostics in CRE Screening
Watch Now
At the 2016 IDWeek meeting in New Orleans, Dr. Teena Chopra delivered a presentation entitled Rapid Diagnostics in the Post Antibiotic Era: Averting a Catastrophe. Click the image above to watch a replay of the full presentation.
This video was made available with the speaker’s permission.
New National Targets for Reducing Healthcare-Associated Infections (HAIs) Announced
The US Department of Health and Human Services (HHS) recently announced new HAI reduction targets for acute care hospitals as part of its national HAI Action Plan. The announcement builds on progress made over the past few years toward goals established using a 2009 baseline. The updated targets are based on a newly established 2015 baseline and represent what the agency called “ambitious but achievable” goals.
Click here to read the full article
Overview of Updated HAI Reduction Targets
Note:Slide the table to see more data
Category |
2020 Target (from 2015 baseline) |
|
|
Active surveillance to reduce MRSA rates and improve outcomes
Watch Now
MRSA is a common healthcare-associated infection, causing hundreds of thousands of infections globally each year. For example, as many as 375,000 MRSA infections and 23,000 related deaths occur annually in the United States alone.1 Implementation of on-demand PCR testing with Xpert MRSA followed by isolation of patients infected or colonized with MRSA has a been shown to reduce both HAI rates and infection-related costs.2
Click here to learn about the integration of on-demand molecular testing into one hospital’s MRSA Active Surveillance Program.
1. Kavanagh KT, Calderon LE, Saman DM and Abusalem SK. The use of surveillance and preventative measures for methicillin-resistant staphylococcus aureus infections in surgical patients. Antimicrob Resist Infect Control. 2014;3:18. ;
2. Spencer M, Barnes S, Parada J et al. A primer on on-demand polymerase chain reaction technology. Am J Infect Control. 2015;43:1102-8.