Threat of Antibiotic Resistance is Greater than Initially Understood
The Centers for Disease Control and Prevention (CDC) 2019 Antibiotic Resistance Threats report shows that more people face antibiotic resistance (AR) than originally understood in the 2013 report. There are more than 2.8 million AR infections occurring in the United States each year. Additionally, there were nearly twice as many annual deaths from AR infections in 2013 than originally reported.1
However, deaths due to AR infections have decreased by 18% since the CDC’s 2013 AR threats report, reflecting the effectiveness of AR reduction efforts.1 The CDC recognizes that simply developing new antibiotics will not address the problem of AR and that diagnostics can be just as critical a tool in the fight against infections because they increase the accuracy and speed of a patient’s diagnosis. Thus, implementing PCR testing improves patient outcomes, decreases length of stay and lowers overall costs.2,3
PCR [testing] cannot only lead to optimal antibiotics that are targeted at the offending bacteria on the day of admission, but also decrease unnecessary empiric antibiotics, which is the current practice. In the patients that we utilized this test on, we were able to enact an intervention that prevented or stopped unnecessary antibiotic use in 100% of the cases.”
– Internist and research fellow at David Geffen School of Medicine at the University of California, Los Angeles
References:
1.Centers for Disease Control and Prevention. More people in the United States dying from antibiotic-resistant infections than previously estimated [press release]. https://www.cdc.gov/media/releases/2019/p1113-antibiotic-resistant.html. Accessed February 16, 2020.
2.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. 43.
3.Rosenthal T. RDTs changing the landscape of ID clinical care. https://www.idse.net/Bacterial-Infections/Article/12-19/RDTs-Changing-the-Landscape-of-ID-Clinical-Care/56651. Published December 19, 2019. Accessed February 16, 2020.
Clinically Significant Association Between CRE Colonization and Bloodstream Infection
Researchers have found support for the strong association between carbapenem-resistant Klebsiella pneumoniae (CRKP) colonization and bloodstream infection (BSI). A recently published study that examined the risk factors for CRKP rectal colonization and subsequent BSI in critically ill patients indicates that hospitalized patients become infected with their colonizing strains.
CRKP strains isolated from both the blood cultures and corresponding rectal specimens of patients were screened by polymerase chain reaction for the presence of antibiotic resistance-associated genes. The blaKPC-2 gene was detected in all tested strains, and over 21% of colonized patients developed a BSI. The duration of intensive care unit (ICU) stay, patient/nurse ratio, and prior use of antimicrobials were associated with CRKP rectal colonization. Limiting antianaerobic antimicrobial administration, reducing the duration of ICU stay, and maintaining a low patient/nurse ratio are possible strategies suggested to restrict rectal CRKP colonization in ICUs. The Centers for Disease Control and Prevention (CDC) has recommended active surveillance testing to identify colonization, limit the spread, and prevent infections.
This is a very aggressive approach but containment of new resistance germs is really important. Otherwise we could see these new nightmare bacteria become very common and essentially untreatable."
Principal Deputy Director of CDC
Kontopoulou K, Iosifidis E, Antoniadou E, et al. The clinical significance of carbapenem-resistant Klebsiella pneumoniae rectal colonization in critically ill patients: from colonization to bloodstream infection. J Med Microbiol. 2019. doi: 10.1099/jmm.0.000921. [Epub ahead of print]
https://www.nbcnews.com/health/health-news/nightmare-bacteria-are-trying-spread-u-s-cdc-says-n862436
Rate of Antibiotic-Resistant Infections Has Doubled in the U.S.
A recent report published in Health Affairs found that the rate of antibiotic-resistant infections has roughly doubled since 2002 in the United States. Rising infection rates add to the costs of health care and compromise the quality of medical and surgical procedures provided.1
Researchers estimated the incremental health care costs of treating a resistant infection and found that antibiotic resistance added $1,383 to the cost of treating a bacterial infection. Using an estimate of the number of such infections in 2014, this amounts to a national cost of $2.2 billion annually.1

The researchers note that given the high additional burden to health care systems, the need for innovative new infection prevention programs, antibiotics, and vaccines to prevent and treat antibiotic-resistant infections is an international priority.1
Faster, more effective diagnostics are another key area of emphasis in the fight against resistance. An analysis supported by the UK Government and Wellcome Trust reported that diagnostics as part of infection prevention programs are an effective, useful tool in fighting antibiotic resistance. Increased use of diagnostics to better inform treatment decisions is not only in patients’ interests, but also in the financial interest of health care systems.2
For material progress to happen over the next five years healthcare systems need to leapfrog to using rapid diagnostics wherever possible, before using an antibiotic.”
References:
1. Thorpe, Kenneth E., et al. “Antibiotic-Resistant Infection Treatment Costs Have Doubled Since 2002, Now Exceeding $2 Billion Annually.” Health Affairs, vol. 37, no. 4, 2018, pp. 662–669., doi:10.1377/hlthaff.2017.1153
2. O’Neill, Jim, et al. The Review on Antimicrobial Resistance. Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics. 2015.
Neisseria gonorrhoeae and Mycoplasma genitalium are evolving into so called superbugs
A recent review article discusses the widespread antimicrobial resistance (AMR) developing in Neisseria gonorrhoeae and Mycoplasma genitalium sexually transmitted infections (STIs).1 The authors describe compounding factors, such as uncontrolled use of antimicrobials and limited surveillance of AMR, likely leading to an increased rate of resistance to essentially all available microbial treatments. In outlining available treatment options for the multi-drug resistant bacteria, focus is placed on effective diagnostics that include frequent testing for AMR in order to guide effective treatment decisions.
The review specifically discusses the pitfalls of syndromic management of nongonococcal urethritis (NGU) in the light of recently updated European Guidelines on NGU and M. genitalium infection.2,3 The updated guidelines call for routine nucleic acid amplification testing (NAAT) to be supplemented with molecular detection of macrolide-resistance-mediating mutations. Knowing the resistance status of an uncomplicated M. genitalium infection (Figure 1) will guide first-line treatment choices towards suitable therapy.
Figure 1: Antimicrobial Therapy of Uncomplicated Mycoplasma genitalium

The current rates of AMR are predicted to persist, if not worsen, due to the high number of infections and the remarkable ability of these organisms to develop resistance. Being familiar with current guidelines on diagnostic and treatment procedures and utilising routine AMR testing will help minimise the development of AMR and substantially improve patient management. Click here for more information on the article in Nature Reviews Urology.1
1. Unemo, M. & Jensen, J.S. ‘Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium’. 2016. Nat. Rev. Urol.268. Published online 10 Jan 2017. doi:10.1038/nrurol
2. Jensen, M. et al. 2016 European guideline on Mycoplasma genitalium infections.
3. Horner, PJ. et al. 2016 European guideline on the management of non-gonococcal urethritis.
Epidemiologists Recommend CRE be Reportable Nationally
The Council of State and Territorial Epidemiologists (CSTE) has released a position paper recommending that all states and territories enact laws to make carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CRE) reportable in their jurisdiction, noting that early detection and aggressive implementation of infection prevention and control strategies are necessary to prevent further spread of CRE.
Specific carbapenemase-producing CRE of concern include Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-β-lactamase (NDM), Verona integron-encoded metallo-β-lactamase (VIM), imipenemase (IMP) metallo-β-lactamase, and oxacillinase-48 (OXA-48). Cepheid’s Xpert Carba-R test is one option identified in the report for rapid detection and differentiation of these resistance mechanisms.
The paper indicates that laboratories should report CRE with all available quantitative and qualitative susceptibility data, results (positive, negative, intermediate) of phenotypic carbapenemase production tests as well as results (positive and negative) of any tests performed for resistance mechanisms (e.g., KPC PCR) to public health authorities. These authorities want to identify all CRE, whether they come from screening or from clinical cultures.
CSTE works to establish more effective relationships among state and other health agencies. It also works to advance public health policy and provides technical advice and assistance to partner organizations and to federal public health agencies such as the Centers for Disease Control and Prevention.
Early detection and aggressive implementation of infection prevention and control strategies are necessary to prevent further spread of CP-CRE.”
CSTE position statement 17-ID-04: Public Health Reporting and National Notification of Carbapenemase Producing Carbapenem-Resistant Enterobacteriaceae (CP-CRE) for E. coli, Klebsiella spp. and Enterobacter spp.
The Cost of an Antibiotic Resistant Outbreak
The costs associated with a nosocomial antibiotic-resistant bacterial outbreak can be substantial, but computing the costs is not always straightforward. Financial burden includes both direct and indirect or opportunity costs, such as reallocated staff time, excess or lost bed utilization, or missed revenue for care that would have been delivered in the absence of an outbreak.
The challenge in estimating these costs was demonstrated in a new study out of the United Kingdom where the costs associated with even a modest carbapenemase-producing Enterobacteriaceae outbreak affecting 40 patients during a 10-month period of time had a significant financial impact: €1.1 million ($1.2 million).1 Since outbreaks can result in significant costs, proactive steps, including patient screening with rapid diagnostic tools, could be employed to minimize the impact.
Table 1: Costs Associated With A CRE Outbreak in the United Kingdom

* Relative costs referenced against the least costly parameter, ward-based monitoring
These figures do not take into account costs for facility improvements, legal expenses, the impact on hospital reputation, or the morbidity and mortality associated with an outbreak. Of the 40 patients affected by the outbreak, 16 died, 5 were discharged with end-of-life care plans, 4 required ongoing hemodialysis, 12 required regular outpatient clinic care, and 3 were discharged from the hospital system.
Click here to read the full journal article from Clinical Microbiology and Infection.
1. Otter JA, Burgess P, Davies F, Mookerjee S, et al. Counting the cost of an outbreak of carbapenemase-producing Enterobacteriaceae: an economic evaluation from a hospital perspective. Clin Microbiol Infect. 2016 Oct 13. pii: S1198-743X(16)30464-5 [Epub ahead of print].
Which States Require CRE Reporting?
With 48 states now reporting confirmed cases of carbapenemase-producing CRE,1 state health departments from Alaska to Florida have implemented CRE reporting requirements. Many of these guidelines, like those from Illinois, Maryland, and Texas, specifically mention PCR tests for KPC, VIM, IMP, NDM, and OXA-48-like carbapenemase genes as important tools for quickly identifying CRE. States like Oregon also point out that the highly transmissible carbapenemase-producing CRE detected by PCR tests are of particular concern and require an institution’s “most aggressive control measures” when identified. A common theme throughout the reporting regulations is the importance of timeliness in the detection and reporting of CRE, with some states requiring notification in as little as 24 hours.
APIC’s Summary of State CRE Reporting Requirements consolidates guidelines from many of these states into a single resource. What does your state require? Remember to follow the links in the document to access the complete and most up-to-date version of each policy.
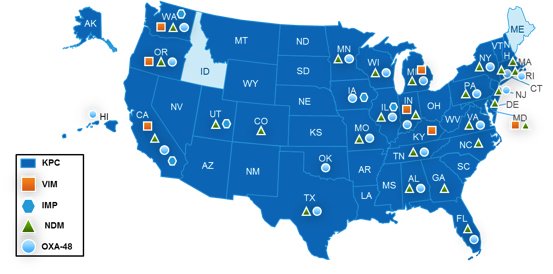
Reported cases of carbapenemase-producing CRE as of April 2016.
Source: http://www.cdc.gov/hai/organisms/cre/TrackingCRE.html updated April 2016.
Tracking Resistance with On-Demand Molecular Diagnostics
Drug resistance is a complex, fast-changing, global health crisis. To fight it, we need collaboration between antimicrobial resistance surveillance networks and centers to learn which pathogens are adapting and where. We need to track each outbreak and work fast to prevent another.1 Surveillance is key—knowledge is power.
Without urgent, coordinated action, the world is heading towards a post-antibiotic era, in which common infections and minor injuries, which have been treatable for decades, can once again kill.”
—World Health Organization, Global Report on Antimicrobial Resistance (2014)
Cepheid provides on-demand testing solutions to help track, manage and reduce the spread of infection.
On-demand PCR tests delivering results in about an hour or less enable real-time monitoring and reporting of pathogens that are critical to infection control and antimicrobial resistance:
1. World Health Organization. “Antimicrobial resistance: global report on surveillance.” June, 2014.
Tracking Disease – Cepheid C360
Watch Now
Cepheid C360 is a secure, hosted platform that collects and aggregates real-time disease and system surveillance information from any GeneXpert® System. Watch our overview video below, and visit www.cepheidc360.com for more information.








